Research
Cambridge University lab grown “mini guts” study
"Cambridge scientists have grown ‘mini-guts’ in the lab to help understand Crohn’s disease, showing that ‘switches’ that modify DNA in gut cells play an important role in the disease and how it presents in patients. These could in future be used to identify the best treatment for an individual patient, allowing for more precise and personalised treatments."

10 news & Garvan Institute of Medical Research segment
A segment on 10 News First that aired yesterday on the Garvan Institute of Medical Research autoimmune disease research project.

Robot surgery for Inflammatory Bowel Disease
Treatment for IBD progresses every year, with the use of biologics reducing the rate of surgery. However, in IBD that does not respond to…

Evaluation of a person-centred communication aid tool for people with Inflammatory Bowel Disease (IBD)
Researchers from the University of Wollongong are recruiting people with Crohn’s disease and colitis for an opportunity to test a new tool designed to support knowledge and confidence building about diet and eating with IBD.

Survey: Sexual and reproductive health needs of women who live in Australia with Inflammatory Bowel Disease.
Researchers at Western Sydney University and Auckland University of Technology want to understand what the sexual and reproductive health needs are of women who live in Australia with the chronic condition of Inflammatory Bowel Disease.

Survey on the sexual and reproductive health needs of women in Australia with IBD
Researchers at Western Sydney University and Auckland University of Technology want to understand what the sexual and reproductive health needs are of women who live in Australia with the chronic condition of Inflammatory Bowel Disease.
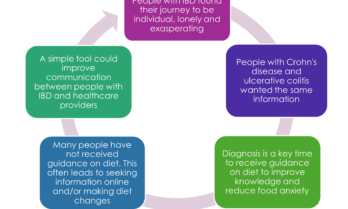
Key findings from IBD and diet research
In 2023, University of Wollongong PhD student Chiara Miglioretto and her supervisors Prof. Kelly Lambert and Prof. Eleanor Beck, completed research into the dietary information needs of people living with IBD, with help from CCA for participants’ recruitment. The findings are now being published and key insights are informing the development of a patient-centred communication aid tool.

Ustekinumab listed on PBS
Ustekinumab is now listed on the PBS for the treatment of complex refractory fistulising Crohn’s disease (fCD).

2024 Scholarship awarded
We are thrilled to announce that the CCA IBD PhD Scholarship of $96,000 over three years has been awarded to Dr Richard Fernandes, University of Queensland. Dr Fernandes is a gastroenterologist who is undertaking the project ‘Improving our understanding of post-operative Crohn’s disease recurrence’

Have your new years resolutions… slipped? Or never got off the ground?
By Gemma Reeves, APD BND BSc (FoodScNut) Health Coach As we gaze at the fireworks in the sky, dream about…

Inflammatory bowel disease associated with atopic dermatitis (eczema)
Inflammatory bowel disease (IBD) has no single cause, with genetics, immune system dysfunction and the gut microbiome all implicated. The…

EEN treatment potential for adults with IBD
The IBD Team at Western Health, Melbourne, have been using Exclusive enteral nutrition (EEN) for several years in adult IBD…